A Long-Standing Legacy Of Protecting The Interests Of Hawaii Residents And Visitors
Since our firm’s inception in 1973, we have provided fierce and successful advocacy after personal injury incidents or medical malpractice errors.
A Long-Standing Legacy Of Protecting The Interests Of Hawaii Residents And Visitors
Since our firm’s inception in 1973, we have provided fierce and successful advocacy after personal injury incidents or medical malpractice errors.
Our Services
The Premier Personal Injury And Medical Malpractice Firm In Hawaii Since 1973
Honolulu Personal Injury And Medical Malpractice Attorneys With A
Commitment To Excellence
Honolulu Personal Injury And Medical Malpractice Attorneys With A
Commitment To Excellence
Our attorneys understand the value of a job well-done and have achieved some of the largest settlements in Hawaii. As a staple of the Honolulu legal community since 1973, we know the intricacies of medical malpractice and personal injury cases and what it takes to win. If you have suffered a serious injury due to another’s negligence, you want to hire a firm whose longevity and reputation speak for itself. At Cronin, Fried, Sekiya, Kekina & Fairbanks, we put our wealth of knowledge and experience to work for you.
Fiercely Advocating For The Injured For Nearly 50 Years
It is no secret that a car crash, truck collision, aviation incident or any other sort of accident can result in drastic, life-changing consequences. In the face of physical, financial and emotional difficulties, life may seem overwhelming. Our Hawaii personal injury lawyers can guide you through every step of the process and handle the complexities of your case, so you can focus on recovery. We can help with coordinating payment of medical expenses, recovering insurance benefits and taking cases to trial as necessary.
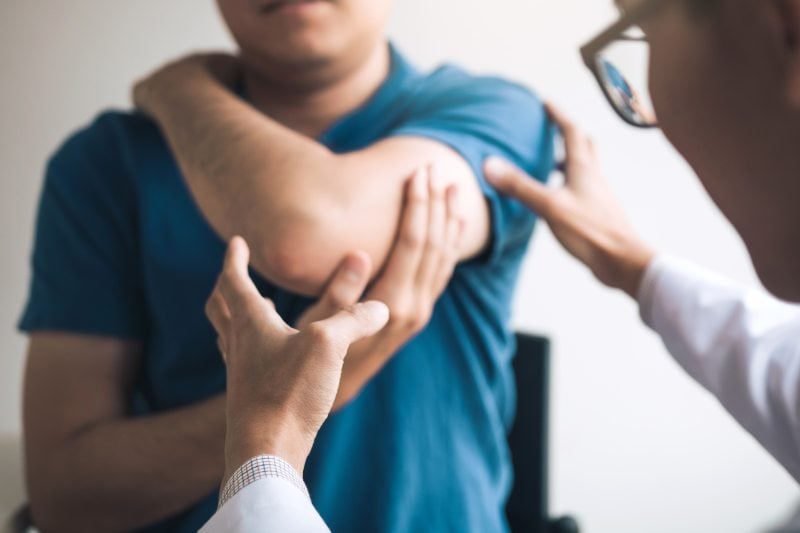
Empowering Victims Of Medical Mistakes
A healthcare professional has a duty to take care of their patients and look out for their interests. When errors happen, victims need a malpractice lawyer who will support them through challenging times and help them fight for the compensation they deserve. Our skilled attorneys at Cronin, Fried, Sekiya, Kekina & Fairbanks can fearlessly stand up for you when you need it the most. We not only concern ourselves with achieving results as quickly as possible, but also with preparing you for your changed future.
To learn more about how we can assist with your medical malpractice or personal injury matter, contact us at 808-600-3514 or fill out our online contact form. From our Honolulu office, we can serve all of Hawaii, as well as visitors from other states.
For a free initial consultation, call us at 808-600-3514.